

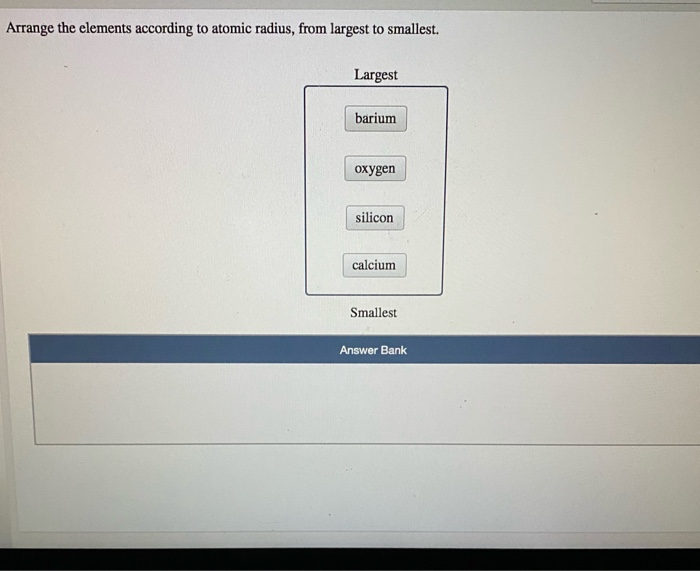
This results in a decrease in atomic radii.Īll of these elements are included in the same period.Īll these facts allow us to arrive at the following order of decreasing atomic radiations:Ībove is the solution of “Arrange these elements according to atomic radius.“. OBJ: Arrange the atoms according to ionization energy (five atoms). As the atomic number increases, so does the effective nuclear charge. Transition elements have larger atomic radii than the preceding IA and IIA elements. The iodine atom is the central element of ICl3 molecular geometry. The lower attraction between nucleus and electrons can cause an increase in the atomic radii. If we place or substitute the values according to the formula, we get Jan 29. The effective nuclear charge is decreased when there are more shells of an atom in a group. This trend can vary depending on the time period or the group. The distance between the nucleus’ inner core and the outermost shell is called the atomic radius. Generally, as we go down in a particular group, we see a rise in the atomic radius. The position of an element in the periodic table determines its atomic radius. Atomic radius increases on moving downwards in a group and decreases on moving in the right in a period from left.
Atomic RadiusĪtomic radius refers to the distance between the nucleus of the nucleus, and the valence shell.
The atomic radius is one of the most important periodic trends. Therefore, increasing order of atomic radii is : F lt N lt Be lt Li(b) The listed elements are present in group 17 (halogen family).
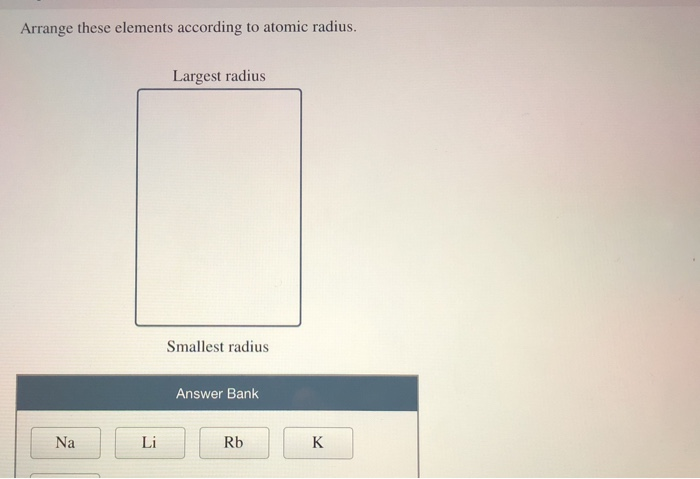
These elements can be either metals or metalloids. The periodic table can be described as a progression in elements according to their increasing atomic number. Rank the following elements by increasing atomic radius: carbon, aluminum, oxygen. Oh businessman Is height that is 213 billion because don't make good ideas increased it. Let’s not forget to look at the content! QuestionĪrrange the elements according to atomic radius Largest radius Ar P S Si Mg Al Na Smallest radius Answer “Arrange these elements according to atomic radius”. By what property does the modern periodic table arrange the elements. VIDEO ANSWER:Time students in the question that given the commissioner during Viktor and BRyce nitrogen are silly hospitals has stayed in business after this dynamic radius. The following article will add knowledge about “ Arrange these elements according to atomic radius.“. Using only the periodic table arrange the following elements in order of increasing atomic radius: bismuth, nitrogen, arsenic, phosphorus Smallest Largest.


 0 kommentar(er)
0 kommentar(er)
